EXPERT VERIFIED Step 1/5 1. The pKa of HCHO2 is 3.74, which means that at pH 3.74, half of the HCHO2 molecules will be in the acid form (HCHO2) and half will be in the conjugate base form (CHO2-). Step 2/5 2. The pH of the HCHO2/NaCHO2 solution is 3.25, which is lower than the pKa of HCHO2.
Solved If the pKa of HCHO2 is 3.74 and the pH of an | Chegg.com
a. law and theory b. theory and… Question Transcribed Image Text: If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NACHO2 solution is 3.11, which of the following is TRUE? O HCHO2] < [NACHO2] [HCHO2] << [NaCHO2] [HCHO2] > [NaCHO2] O HCHO2] = [NACHO2] O It is not possible to make a buffer of this pH from HCHO2 and NaCHO2. %3D Expert Solution
Source Image: coursehero.com
Download Image
Video Transcript. Hi in this question, we have given h c h, o 2 p c value is 3.74 and ph of h, c h, o 2 n, a c h, o 2 solution is 4.02. We need to find out what would be the correct option for h, c h, o 2 and a c h, o 2 concentration.
Source Image: chegg.com
Download Image
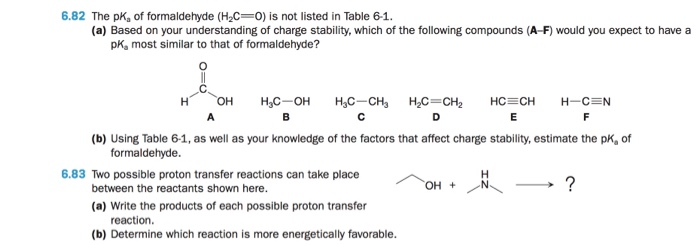
Solved 6.82 The pKa of formaldehyde (H2C- O) is not listed | Chegg.com If the pKa of HCHO₂ is 3.74 and the pH of an HCHO₂/NaCHO₂ solution is 3.11, then the statement which is correct is [HCHO₂] > [NaCHO₂].. Buffer solution is a solution which is a mixture of weak acid and its conjugate base or vice a versa. It shows a very little change in pH when strong acid or base is added in it.. Since NaCHO₂ is a basic salt of formic acid and it acts as a buffer
Source Image: chegg.com
Download Image
If The Pka Of Hcho2 Is 3.74
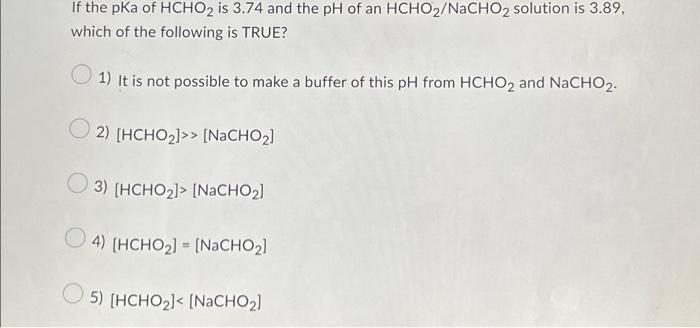
If the pKa of HCHO₂ is 3.74 and the pH of an HCHO₂/NaCHO₂ solution is 3.11, then the statement which is correct is [HCHO₂] > [NaCHO₂].. Buffer solution is a solution which is a mixture of weak acid and its conjugate base or vice a versa. It shows a very little change in pH when strong acid or base is added in it.. Since NaCHO₂ is a basic salt of formic acid and it acts as a buffer Chemistry Chemistry questions and answers If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.89, which of the following is TRUE? 1) It is not possible to make a buffer of this pH from HCHO2 and NaCHO2. 2) [HCHO₂]> > [NaCHO₂] 3) [HCHO₂]> [NaCHO₂] 4) [HCHO₂] = [NaCHO₂] 5) [HCHO₂]< [NaCHO₂] This problem has been solved!
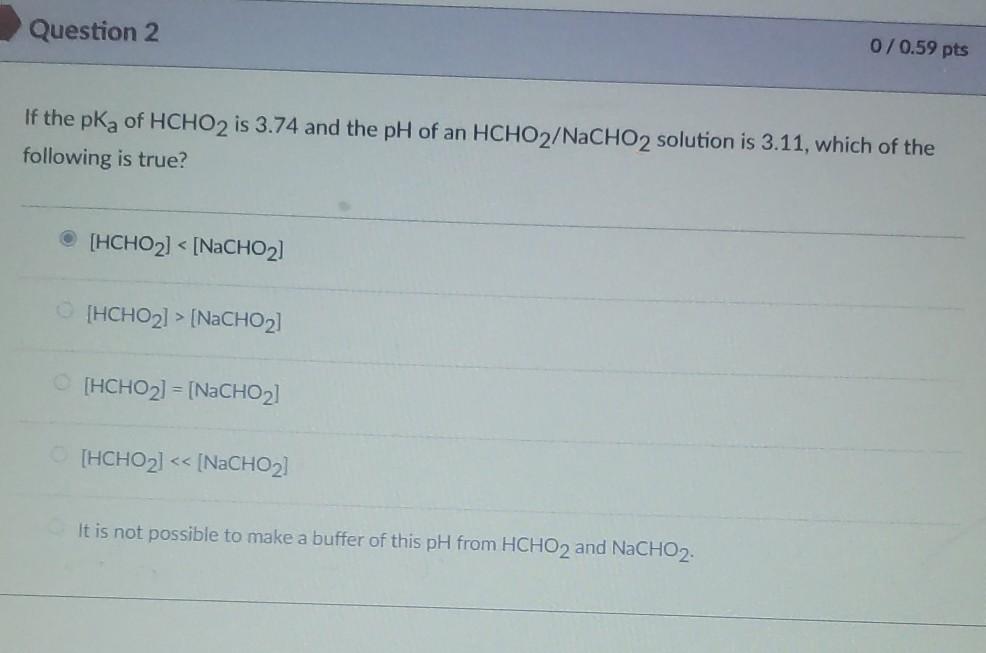
Solved Question 2 0/0.59 pts If the pKa of HCHO2 is 3.74 and | Chegg.com
May 24, 2023High School answered • expert verified If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.11, which of the following is TRUE? a) [HCHO2] < [NaCHO2] b) [HCHO2] = [NaCHO2] c) [HCHO2] << [NaCHO2] d) [HCHO2] > [NaCHO2] e) It is not possible to make a buffer of this pH from HCHO2 and NaCHO2 Solved If the pKa of HCHO2 is 3.74 and the pH of an | Chegg.com
Source Image: chegg.com
Download Image
Solved 6) If the pKa of HCHO2 is 3.74 and the pH of an | Chegg.com May 24, 2023High School answered • expert verified If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.11, which of the following is TRUE? a) [HCHO2] < [NaCHO2] b) [HCHO2] = [NaCHO2] c) [HCHO2] << [NaCHO2] d) [HCHO2] > [NaCHO2] e) It is not possible to make a buffer of this pH from HCHO2 and NaCHO2

Source Image: chegg.com
Download Image
Solved If the pKa of HCHO2 is 3.74 and the pH of an | Chegg.com EXPERT VERIFIED Step 1/5 1. The pKa of HCHO2 is 3.74, which means that at pH 3.74, half of the HCHO2 molecules will be in the acid form (HCHO2) and half will be in the conjugate base form (CHO2-). Step 2/5 2. The pH of the HCHO2/NaCHO2 solution is 3.25, which is lower than the pKa of HCHO2.
Source Image: chegg.com
Download Image
Solved 6.82 The pKa of formaldehyde (H2C- O) is not listed | Chegg.com Video Transcript. Hi in this question, we have given h c h, o 2 p c value is 3.74 and ph of h, c h, o 2 n, a c h, o 2 solution is 4.02. We need to find out what would be the correct option for h, c h, o 2 and a c h, o 2 concentration.
Source Image: chegg.com
Download Image
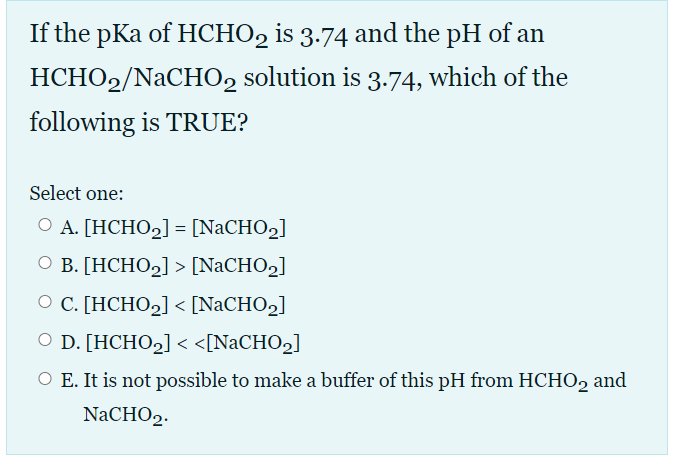
ch17 PDF | PDF | Acid | Titration A) [HCHO2] < [NaCHO2] B) [HCHO2] = [NaCHO2] C) [HCHO2] > [NaCHO2] D) [HCHO2] >> [NaCHO2] E) It is not possible to make a buffer of this pH from HCHO2 and NaCHO2. A) [HCHO2] < [NaCHO2] If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.74, which of the following is TRUE?

Source Image: scribd.com
Download Image
8.2 Strong and Weak Acids and Bases – ppt video online download If the pKa of HCHO₂ is 3.74 and the pH of an HCHO₂/NaCHO₂ solution is 3.11, then the statement which is correct is [HCHO₂] > [NaCHO₂].. Buffer solution is a solution which is a mixture of weak acid and its conjugate base or vice a versa. It shows a very little change in pH when strong acid or base is added in it.. Since NaCHO₂ is a basic salt of formic acid and it acts as a buffer

Source Image: slideplayer.com
Download Image
General Chemistry 2: Quarter 4 – Module 4: PH and Buffer Solutions | PDF | Ph | Buffer Solution Chemistry Chemistry questions and answers If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.89, which of the following is TRUE? 1) It is not possible to make a buffer of this pH from HCHO2 and NaCHO2. 2) [HCHO₂]> > [NaCHO₂] 3) [HCHO₂]> [NaCHO₂] 4) [HCHO₂] = [NaCHO₂] 5) [HCHO₂]< [NaCHO₂] This problem has been solved!

Source Image: scribd.com
Download Image
Solved 6) If the pKa of HCHO2 is 3.74 and the pH of an | Chegg.com
General Chemistry 2: Quarter 4 – Module 4: PH and Buffer Solutions | PDF | Ph | Buffer Solution a. law and theory b. theory and… Question Transcribed Image Text: If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NACHO2 solution is 3.11, which of the following is TRUE? O HCHO2] < [NACHO2] [HCHO2] << [NaCHO2] [HCHO2] > [NaCHO2] O HCHO2] = [NACHO2] O It is not possible to make a buffer of this pH from HCHO2 and NaCHO2. %3D Expert Solution
Solved 6.82 The pKa of formaldehyde (H2C- O) is not listed | Chegg.com 8.2 Strong and Weak Acids and Bases – ppt video online download A) [HCHO2] < [NaCHO2] B) [HCHO2] = [NaCHO2] C) [HCHO2] > [NaCHO2] D) [HCHO2] >> [NaCHO2] E) It is not possible to make a buffer of this pH from HCHO2 and NaCHO2. A) [HCHO2] < [NaCHO2] If the pKa of HCHO2 is 3.74 and the pH of an HCHO2/NaCHO2 solution is 3.74, which of the following is TRUE?