1 Citations 2 Altmetric Metrics Retrosynthetic analysis is the way that organic chemists draw an imaginary line from a target molecule to available precursors. In addition to functional group
Solved] Chem Question. Consider the retrosynthesis of the following target… | Course Hero
inspired by how chemists approach retrosynthesis prediction. Our method disas-sembles retrosynthesis into two steps: i) identify the potential reaction center of the target molecule through a novel graph neural network and generate intermedi-ate synthons, and ii) generate the reactants associated with synthons via a robust reactant generation
Source Image: kpu.pressbooks.pub
Download Image
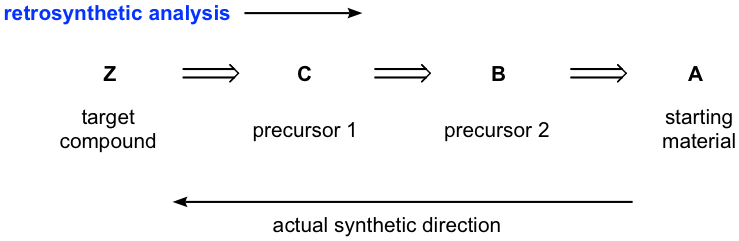
the molecule to be synthesized. Retrosynthetic analysis or retrosynthesis. the process of mentally breaking down a molecule into a starting material. Disconnection. an imaginary bond cleavage corresponding to a reverse of a real reaction. Transform. the exact reverse of a synthetic reaction. Retron.

Source Image: target.com
Download Image
Propose a method to synthesize these target molecules from the specified starting materials. For each target molecule, provide both a retrosynthetic analysis and a forward synthetic scheme. Be sure to | Homework.Study.com 9.6 Synthesis of Target Molecules: Introduction to Retrosynthetic Analysis So far, we have learned three major types of reactions: nucleophilic substitution, elimination and the halogenation of alkane (radical substitution).

Source Image: chegg.com
Download Image
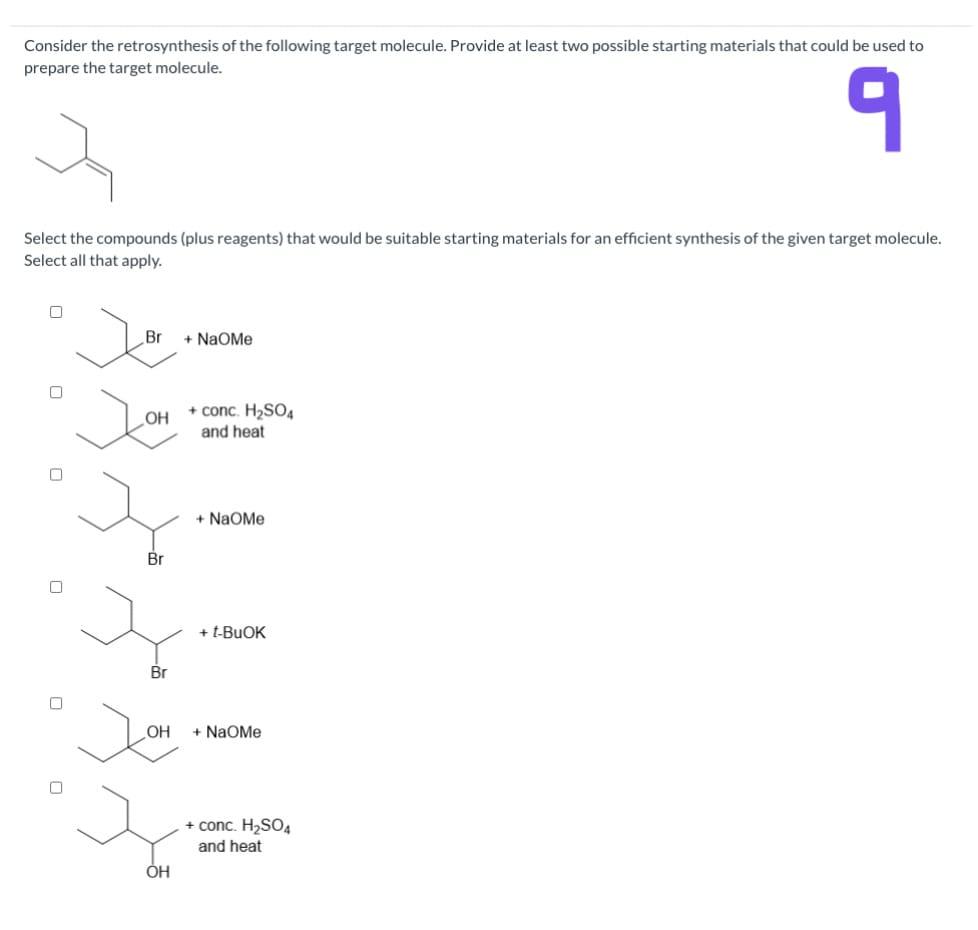
Consider The Retrosynthesis Of The Following Target Molecule
9.6 Synthesis of Target Molecules: Introduction to Retrosynthetic Analysis So far, we have learned three major types of reactions: nucleophilic substitution, elimination and the halogenation of alkane (radical substitution). Each plan thus evolved, describes a ‘ROUTE’ based on a retrosynthesis. Each disconnection leads to a simplified structure. The logic of such disconnections forms the basis for the retroanalysis of a given target molecule. Natural products have provided chemists with a large variety of structures, having complex functionalities and
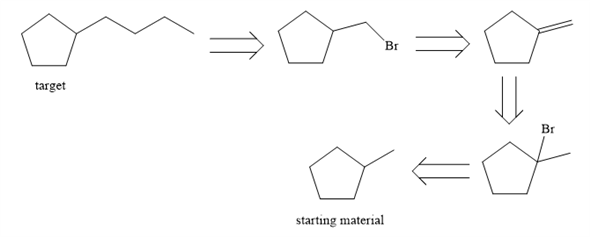
Solved I must perform a retrosynthesis of the target | Chegg.com
Jul 20, 2022Retrosynthetic analysis – the concept of mentally dismantling a molecule step by step all the way back to smaller, simpler precursors using known reactions – is a powerful and widely-used intellectual tool first developed by synthetic organic chemists. Solved: Chapter 16CA.PC Problem 45CTQ Solution | Organic Chemistry 2nd Edition | Chegg.com
Source Image: chegg.com
Download Image
Solved ( give answer with mechanism , more than one option | Chegg.com Jul 20, 2022Retrosynthetic analysis – the concept of mentally dismantling a molecule step by step all the way back to smaller, simpler precursors using known reactions – is a powerful and widely-used intellectual tool first developed by synthetic organic chemists.
Source Image: chegg.com
Download Image
Solved] Chem Question. Consider the retrosynthesis of the following target… | Course Hero 1 Citations 2 Altmetric Metrics Retrosynthetic analysis is the way that organic chemists draw an imaginary line from a target molecule to available precursors. In addition to functional group
Source Image: coursehero.com
Download Image
Propose a method to synthesize these target molecules from the specified starting materials. For each target molecule, provide both a retrosynthetic analysis and a forward synthetic scheme. Be sure to | Homework.Study.com the molecule to be synthesized. Retrosynthetic analysis or retrosynthesis. the process of mentally breaking down a molecule into a starting material. Disconnection. an imaginary bond cleavage corresponding to a reverse of a real reaction. Transform. the exact reverse of a synthetic reaction. Retron.

Source Image: homework.study.com
Download Image
Revolutionary Automated Method Predicts Stereochemistry of Pericyclic Reactions Retrosynthesis is the process of “deconstructing” a target molecule into readily available starting materials by means of – imaginary breaking of bonds (disconnections) and by the conversion of one functional group into another (functional group interconversions). The following factors need be taken into consideration:

Source Image: scitechdaily.com
Download Image
Large-Scale Distributed Training of Transformers for Chemical Fingerprinting | Journal of Chemical Information and Modeling 9.6 Synthesis of Target Molecules: Introduction to Retrosynthetic Analysis So far, we have learned three major types of reactions: nucleophilic substitution, elimination and the halogenation of alkane (radical substitution).

Source Image: pubs.acs.org
Download Image
Reversing oncogenic transformation with iron chelation | Oncotarget Each plan thus evolved, describes a ‘ROUTE’ based on a retrosynthesis. Each disconnection leads to a simplified structure. The logic of such disconnections forms the basis for the retroanalysis of a given target molecule. Natural products have provided chemists with a large variety of structures, having complex functionalities and
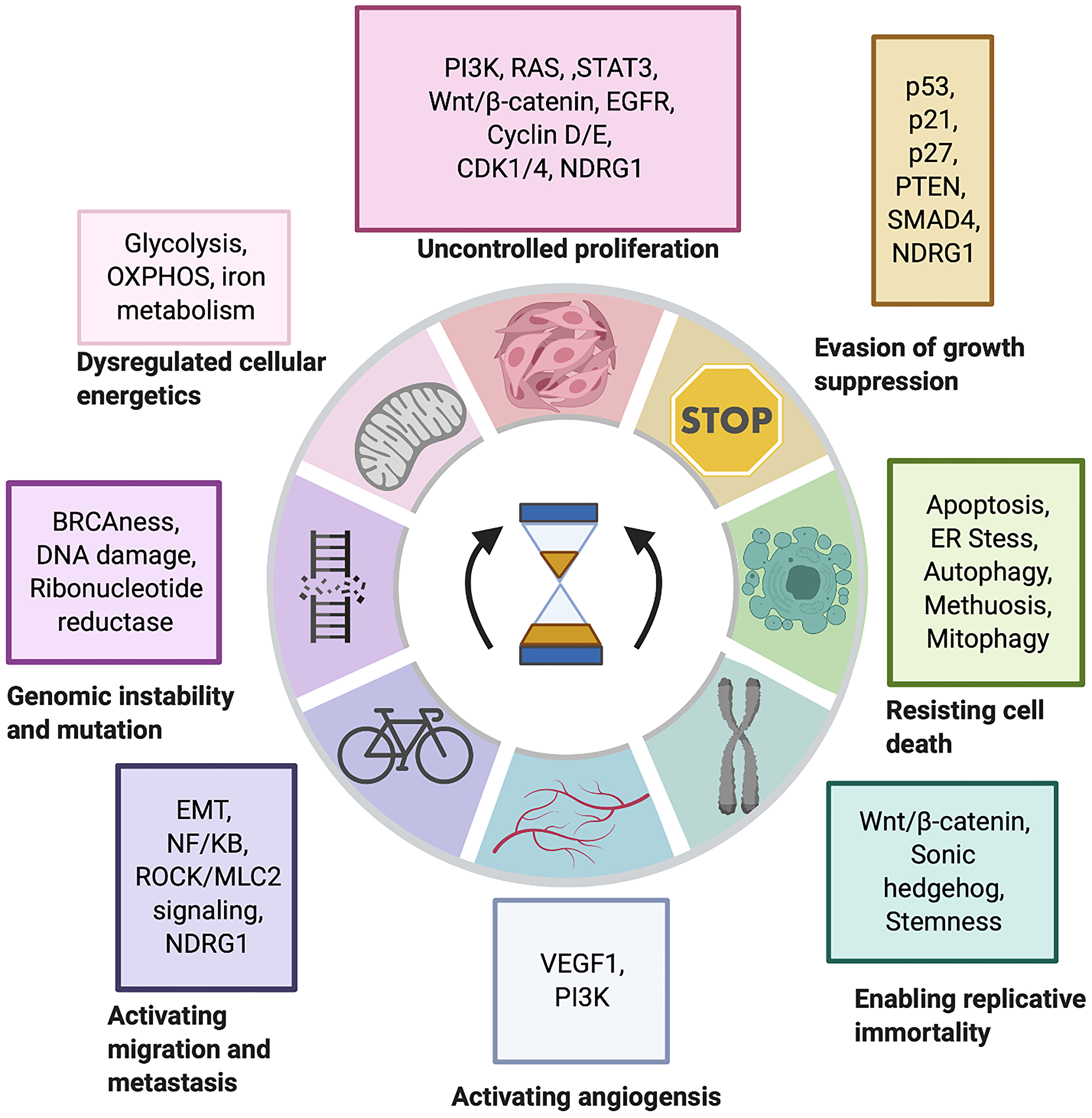
Source Image: oncotarget.com
Download Image
Solved ( give answer with mechanism , more than one option | Chegg.com
Reversing oncogenic transformation with iron chelation | Oncotarget inspired by how chemists approach retrosynthesis prediction. Our method disas-sembles retrosynthesis into two steps: i) identify the potential reaction center of the target molecule through a novel graph neural network and generate intermedi-ate synthons, and ii) generate the reactants associated with synthons via a robust reactant generation
Propose a method to synthesize these target molecules from the specified starting materials. For each target molecule, provide both a retrosynthetic analysis and a forward synthetic scheme. Be sure to | Homework.Study.com Large-Scale Distributed Training of Transformers for Chemical Fingerprinting | Journal of Chemical Information and Modeling Retrosynthesis is the process of “deconstructing” a target molecule into readily available starting materials by means of – imaginary breaking of bonds (disconnections) and by the conversion of one functional group into another (functional group interconversions). The following factors need be taken into consideration: