Sep 22, 2022Introductory Chemistry Online! (Young) 3: Chemical Bonding and Nomenclature 3.4: Identifying Molecular and Ionic Compounds
Solved Classify each compound as an ionic compound or a | Chegg.com
Common salt (sodium chloride) is one of the best-known ionic compounds. Molecular compounds contain discrete molecules, which are held together by sharing electrons ( covalent bonding). Examples are water, which contains H 2 O molecules; methane, which contains CH 4 molecules; and hydrogen fluoride, which contains HF molecules.

Source Image: pinterest.com
Download Image
Explain how to name an acid. ending ide add ic acid Ending ite add ous acid ending in ate add ic acid. Classify each compound as ionic or molecular as their basic unit a) Co2 b) NiCl2 c) NaI d) PCl3. a molecular b ionic c ionic d molecular. Classify each compound as ionic or molecular a) CF2Cl2 b) CCl4 c) PtO2 d) SO3.

Source Image: in.pinterest.com
Download Image
13 Crystal Structures – Mineralogy Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals.

Source Image: scribd.com
Download Image
Classify Each Compound As Ionic Or Molecular
Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals. Some elements exist naturally as molecules. For example, hydrogen and oxygen exist as two-atom molecules. Other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (Table \(\PageIndex1\)). As with any molecule, these elements are labeled with a molecular formula, a formal listing of what and how many atoms are in a molecule.
Unit 2 Notes – Molecular & Ionic Compound Structure & Properties | PDF | Ionic Bonding | Chemical Bond
Study with Quizlet and memorize flashcards containing terms like Classify these compounds as ionic or covalent: KCl Ca(NO3)2 CBr4 AlBr3 Br2 NO2 NH3 NaNO2, Ion formation: _____ tend to lose electrons to form _____ charged Ions, and _____ tend to gain electrons to form _____ charged ions., A positively charged ion is known as a(n) _____ and a negatively charge ion is known as a(n) _____. and more. PPT – Chapter 5 – Molecules and Compounds PowerPoint Presentation, free download – ID:4567006
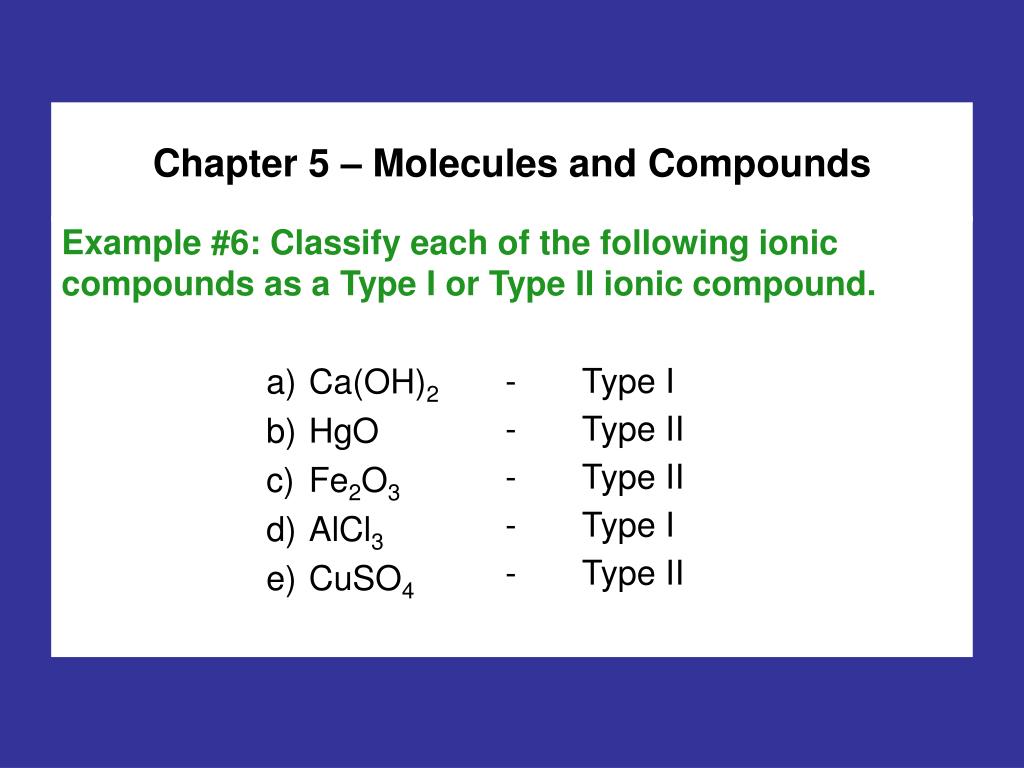
Source Image: slideserve.com
Download Image
Solved Classify each of these chemical compounds: compound | Chegg.com Study with Quizlet and memorize flashcards containing terms like Classify these compounds as ionic or covalent: KCl Ca(NO3)2 CBr4 AlBr3 Br2 NO2 NH3 NaNO2, Ion formation: _____ tend to lose electrons to form _____ charged Ions, and _____ tend to gain electrons to form _____ charged ions., A positively charged ion is known as a(n) _____ and a negatively charge ion is known as a(n) _____. and more.
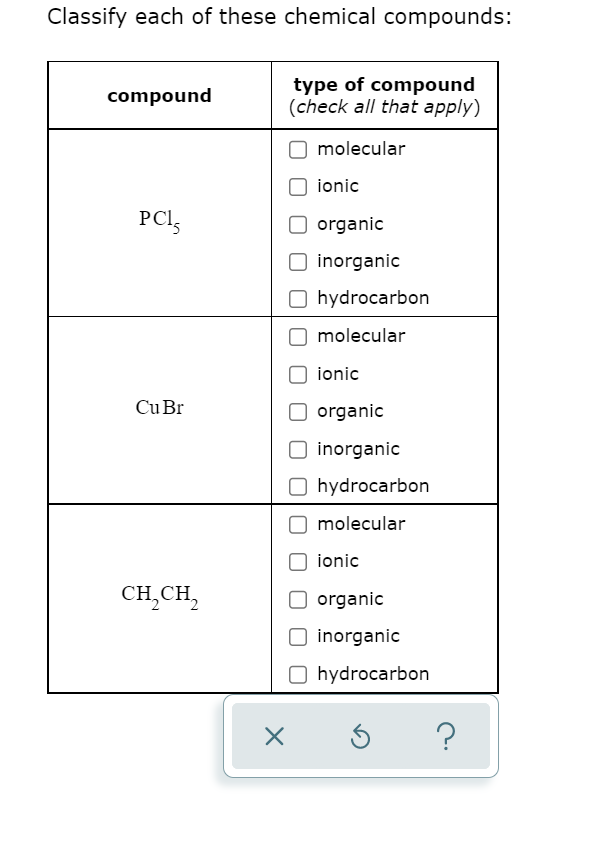
Source Image: chegg.com
Download Image
Solved Classify each compound as an ionic compound or a | Chegg.com Sep 22, 2022Introductory Chemistry Online! (Young) 3: Chemical Bonding and Nomenclature 3.4: Identifying Molecular and Ionic Compounds
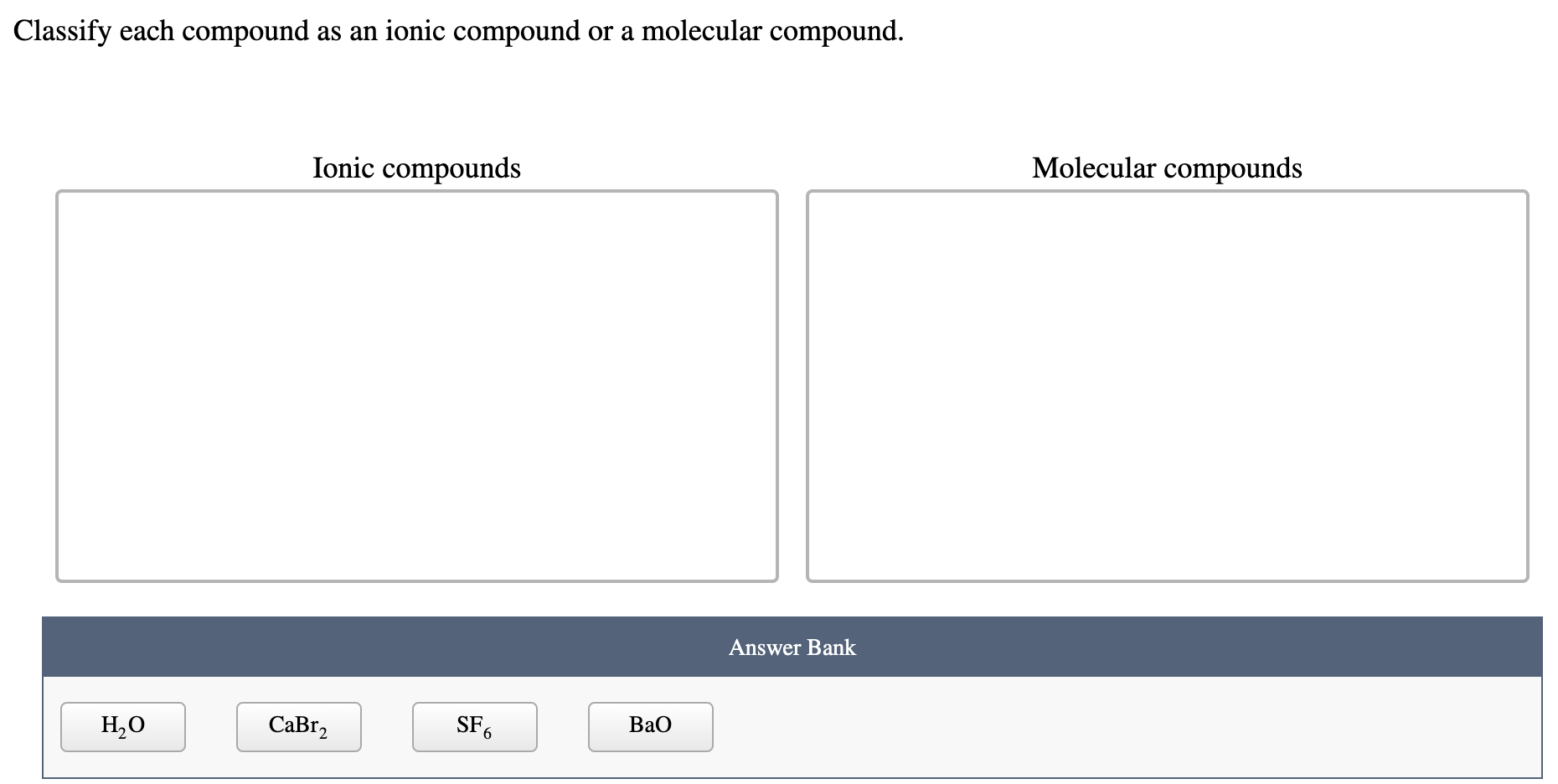
Source Image: chegg.com
Download Image
13 Crystal Structures – Mineralogy Explain how to name an acid. ending ide add ic acid Ending ite add ous acid ending in ate add ic acid. Classify each compound as ionic or molecular as their basic unit a) Co2 b) NiCl2 c) NaI d) PCl3. a molecular b ionic c ionic d molecular. Classify each compound as ionic or molecular a) CF2Cl2 b) CCl4 c) PtO2 d) SO3.
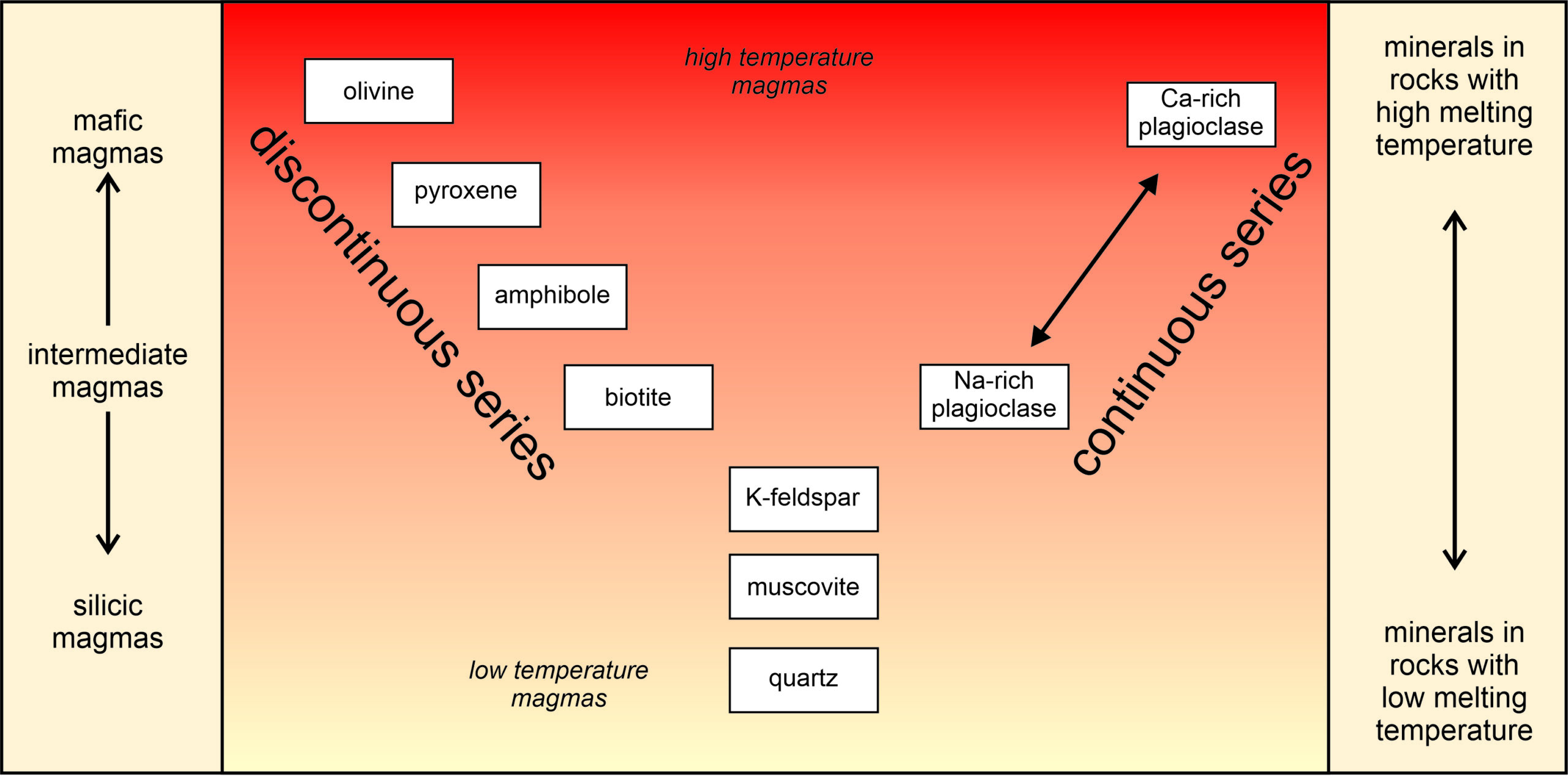
Source Image: opengeology.org
Download Image
Solved Classify each chemical compound: compound type of | Chegg.com SF 6 is a binary molecular compound. SF 6 has two elements, which makes it binary. S and F are both nonmetals, making the compound molecular. Answer E Na 2 SO 4 is an ionic compound containing a polyatomic ion. Na 2 SO 4 has three elements, so it cannot be binary. Na is a metal and both S and O are nonmetals, making the compound ionic. Answer F

Source Image: chegg.com
Download Image
Solved Classify each chemical compound: compound type of | Chegg.com Under normal conditions, molecular compounds often exist as gases, low-boiling liquids, and low-melting solids, although many important exceptions exist. Whereas ionic compounds are usually formed when a metal and a nonmetal combine, covalent compounds are usually formed by a combination of nonmetals.
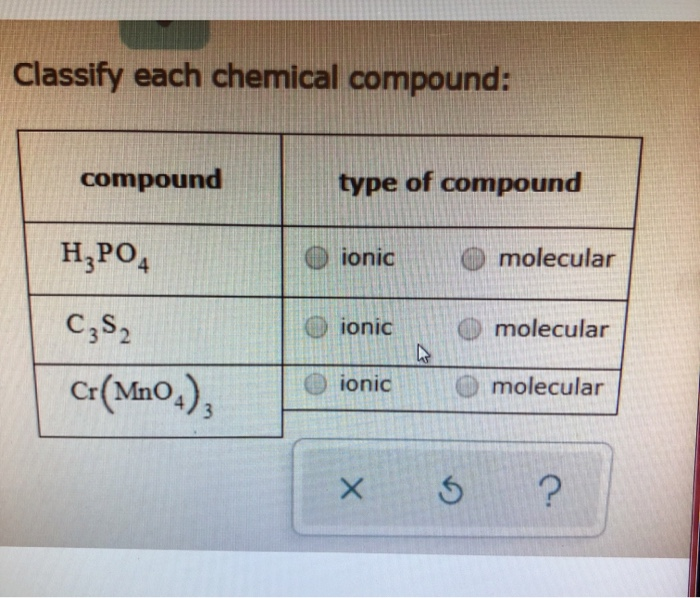
Source Image: chegg.com
Download Image
Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review Some elements exist naturally as molecules. For example, hydrogen and oxygen exist as two-atom molecules. Other elements also exist naturally as diatomic molecules—a molecule with only two ato ms (Table \(\PageIndex1\)). As with any molecule, these elements are labeled with a molecular formula, a formal listing of what and how many atoms are in a molecule.

Source Image: degruyter.com
Download Image
Solved Classify each of these chemical compounds: compound | Chegg.com
Antimicrobial activities of the extracts and secondary metabolites from Clausena genus – A review Common salt (sodium chloride) is one of the best-known ionic compounds. Molecular compounds contain discrete molecules, which are held together by sharing electrons ( covalent bonding). Examples are water, which contains H 2 O molecules; methane, which contains CH 4 molecules; and hydrogen fluoride, which contains HF molecules.
13 Crystal Structures – Mineralogy Solved Classify each chemical compound: compound type of | Chegg.com SF 6 is a binary molecular compound. SF 6 has two elements, which makes it binary. S and F are both nonmetals, making the compound molecular. Answer E Na 2 SO 4 is an ionic compound containing a polyatomic ion. Na 2 SO 4 has three elements, so it cannot be binary. Na is a metal and both S and O are nonmetals, making the compound ionic. Answer F